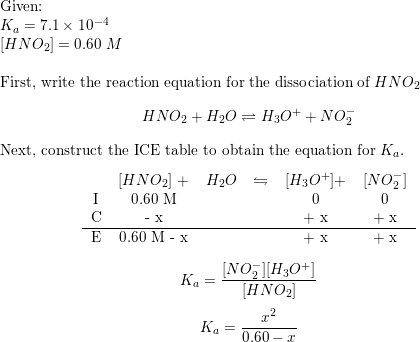Kuchh To LOGO Kahega , LOGO Ka Kaam Hai Kehna: A guide From Ideation -> Making -> Registration of your brand LOGO , For Startups, Budding Entrepreneurs & Students. eBook : Vora,
Two springs A and B having spring constant KA and KB (KA = 2KB) are stretched by applying force of equal magnitude. If energy stored in spring A is EA then energy
![For the reaction: 2A + B → A2B , the rate = k[A][B]^2 with k = 2.0 × 10^-6 mol^-2 L^2 s^-1 . Calculate the initial rate of the reaction when [A] = For the reaction: 2A + B → A2B , the rate = k[A][B]^2 with k = 2.0 × 10^-6 mol^-2 L^2 s^-1 . Calculate the initial rate of the reaction when [A] =](https://d1hhj0t1vdqi7c.cloudfront.net/v1/TVhJa2FqVWNSMFk=/sd/)
For the reaction: 2A + B → A2B , the rate = k[A][B]^2 with k = 2.0 × 10^-6 mol^-2 L^2 s^-1 . Calculate the initial rate of the reaction when [A] =

Budget: Expectations from the Azadi ka Amrit Mahotsav Budget: Creating a land of startups, not subsidiaries - The Economic Times

Question Video: Calculating 𝐾_𝐚 given the Concentration and the Percentage of Dissociation of an Acid | Nagwa