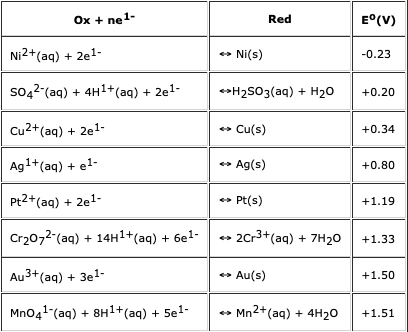
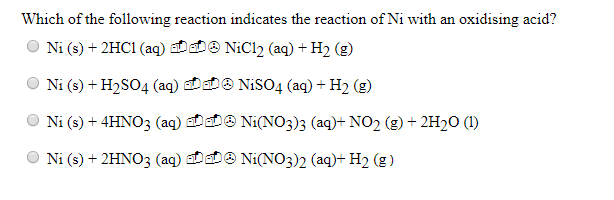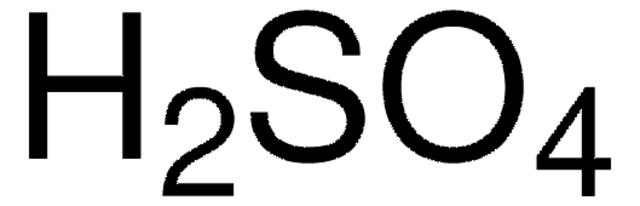How to balance Ni+H2SO4=Ni2(SO4)3+H2|Chemical equation Ni+H2SO4=Ni2(SO4)3+H2| Ni+H2SO4=Ni2(SO4)3+H2 - YouTube
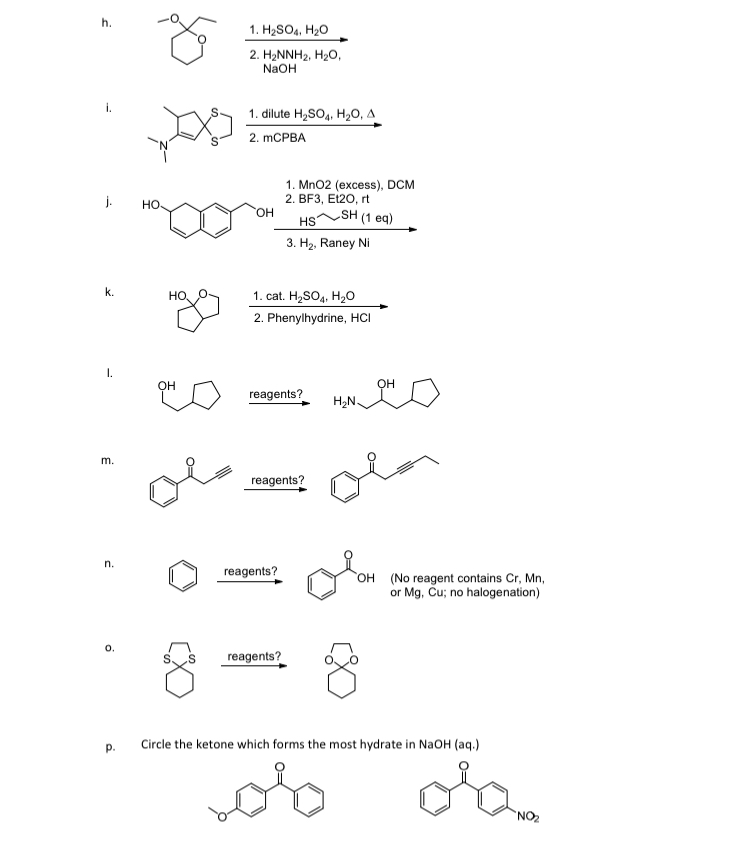
![4] Complete and balance the following organic reactions (ii) to (v) and match the names of each reaction from list (2) to (5). One has been done you as an example. Conc. 4] Complete and balance the following organic reactions (ii) to (v) and match the names of each reaction from list (2) to (5). One has been done you as an example. Conc.](https://toppr-doubts-media.s3.amazonaws.com/images/4903703/da785a60-cf16-4f6b-8427-bb073f7a8246.jpg)
4] Complete and balance the following organic reactions (ii) to (v) and match the names of each reaction from list (2) to (5). One has been done you as an example. Conc.

Comparison between experimental and calculated I-E curves. Cu-Ni in 0.5 M H2SO4 + 10 −4 M cysteine: cathodic scan (cf. Fig. 2).

Leaching Kinetics of Mo, Ni, and Al Oxides from Spent Nickel–Molybdenum Hydrodesulfurization Catalyst in H2SO4 Solution | SpringerLink

Synergistic Recovery of Valuable Metals from Spent Nickel–Metal Hydride Batteries and Lithium-Ion Batteries | ACS Sustainable Chemistry & Engineering

Carbon-supported Ni(OH)2 nanospheres decorated with Au nanoparticles: a promising catalyst for BH4− electrooxidation | Ionics

Leaching Kinetics of Mo, Ni, and Al Oxides from Spent Nickel–Molybdenum Hydrodesulfurization Catalyst in H2SO4 Solution | SpringerLink

A Little Nickel Goes a Long Way: Ni Incorporation into Rh2P for Stable Bifunctional Electrocatalytic Water Splitting in Acidic Media | ACS Materials Au
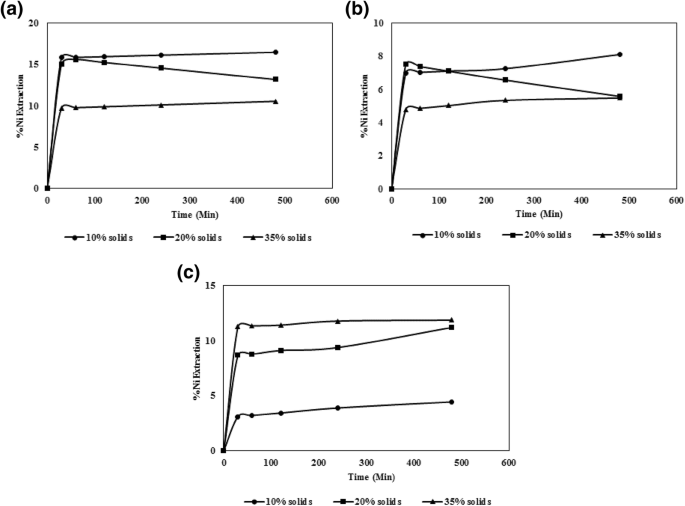
Characteristics of Leaching of Nickel from a Mafic Overburden in Sulfuric Acid and Sodium Chloride Medium at Atmospheric Pressure | SpringerLink
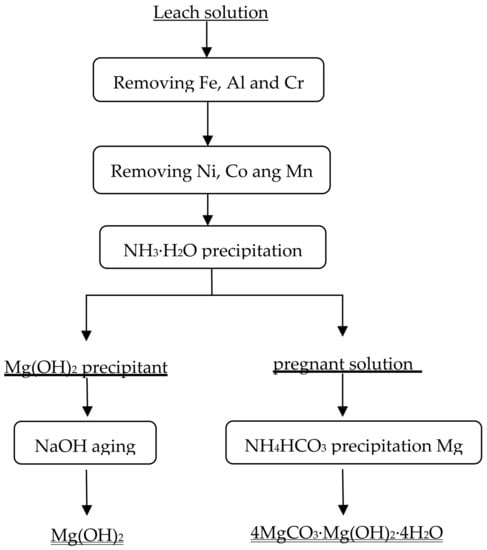
Minerals | Free Full-Text | Recovery of Mg from H2SO4 Leaching Solution of Serpentine to Precipitation of High-Purity Mg(OH)2 and 4MgCO3·Mg(OH)2·4H2O

Green separation and recovery of cobalt and nickel from sulphuric acid achieved by complexation-assisted solvent extraction - ScienceDirect
![SOLVED: Fe(OH)3, Co(OH)3, Ni(OH)2, MnO2 REACTION WITH [H2SO4, H2O2] HOW TO GET Fe3+ Co2+ Ni2+ Mn2+ JUST WRITE NET IONIC EQUATION FOR 4 OF THEM SOLVED: Fe(OH)3, Co(OH)3, Ni(OH)2, MnO2 REACTION WITH [H2SO4, H2O2] HOW TO GET Fe3+ Co2+ Ni2+ Mn2+ JUST WRITE NET IONIC EQUATION FOR 4 OF THEM](https://cdn.numerade.com/ask_previews/8be369ab-e2f0-4054-a35e-d3e0c372f22f_large.jpg)
SOLVED: Fe(OH)3, Co(OH)3, Ni(OH)2, MnO2 REACTION WITH [H2SO4, H2O2] HOW TO GET Fe3+ Co2+ Ni2+ Mn2+ JUST WRITE NET IONIC EQUATION FOR 4 OF THEM