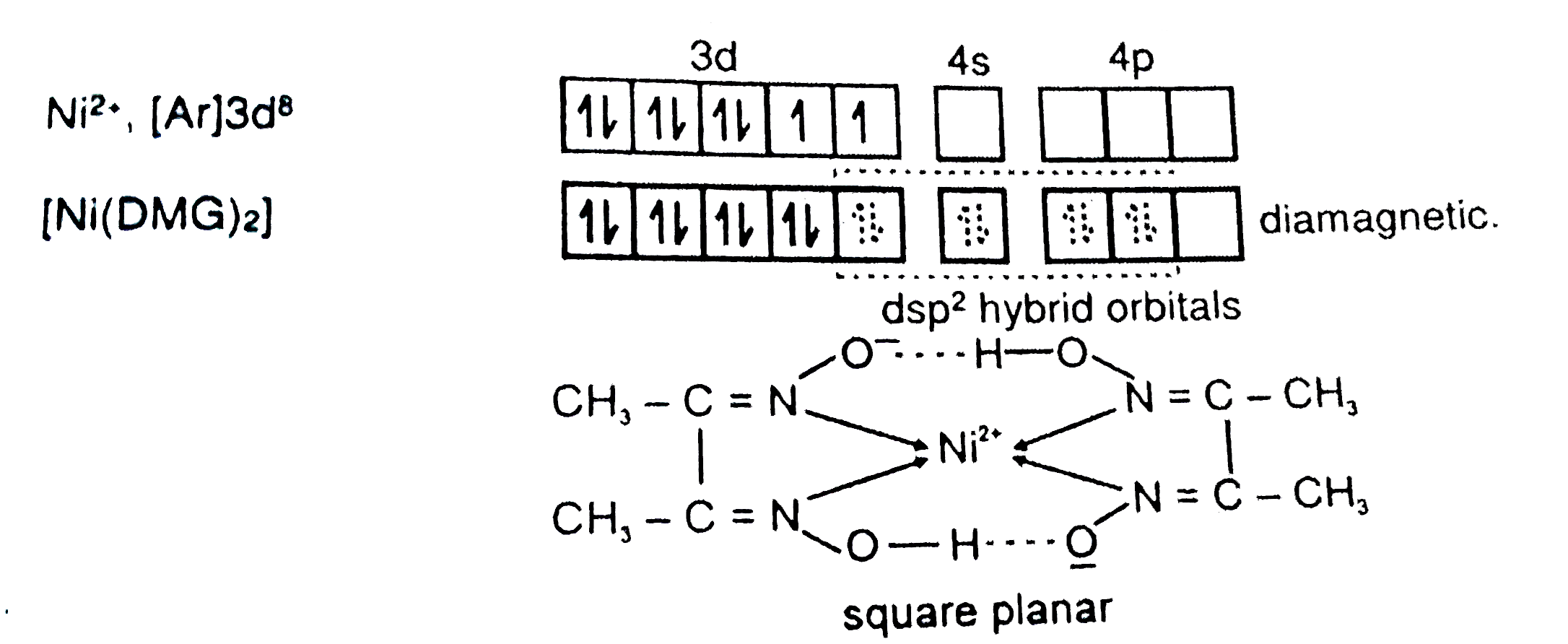
27. DMG +NiCl2+NH4OH makes Complex a+ NH4Cl+H2O. What is complex a and find the hybridisation magnetic character and Oxidation state of Nickel in complex a ?

Separation of Ni, Co, and Mn from Spent LiNi0.5Mn0.3Co0.2O2 Cathode Materials by Ammonia Dissolution | ACS Sustainable Chemistry & Engineering

E740: Equilibrium – Complex Ions – Metal + Ammonia Complexes | Lecture Demonstration Manual General Chemistry | University of Colorado Boulder
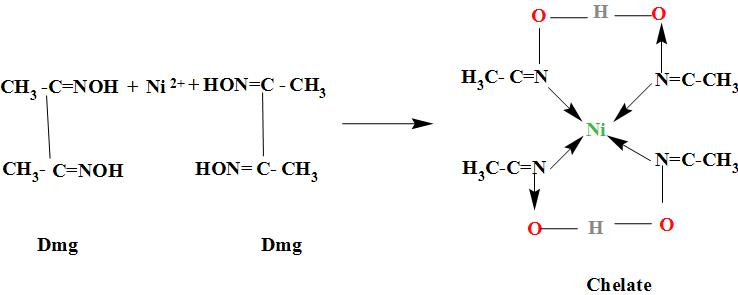
When dimethylglyoxime solution is added to an aqueous solution of nickel (II) chloride followed by ammonium hydroxide, then which of the following statements are incorrect?This question has multiple correct questions(a) No precipitate

E735: Complex Ions and Precipitates – Nickel(II) compounds | Lecture Demonstration Manual General Chemistry | University of Colorado Boulder
What is nh3 aqueous solution?. NH3 aqueous means aqueous solution of… | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium

Effect of temperature on Ni and Cd leaching recovery (NH4OH / (NH4)2CO3... | Download Scientific Diagram

It is an experimental fact that:- DMG+Ni(II) salt+NH_4OH→ Red precipitate Which of the following ... - YouTube

Shape-controlled synthesis of Ni(OH)2/NiO nanowalls by surface reaction of Ni foil in aqueous NH4OH - ScienceDirect

Epitaxial N–H-doped Ni1−x O and Ni2O3 with special planar defects by pulsed laser ablation of metallic Ni in aqueous ammonia | Applied Physics A


/NH4OH%20Fusion%20Chemical%20Blending%20System.jpg?width=1305&name=NH4OH%20Fusion%20Chemical%20Blending%20System.jpg)